While I was in Harrisburg, PA for the Arthritis Foundation’s Advocacy Day this year, one of our ‘asks’ revolved around the development of biosimilars.
I’d be hard pressed to find an autoimmune patient who doesn’t know what a biologic is, but many of them aren’t as familiar with what a biosimilar is. My first reaction to hearing about biosimilars was negative. How could insurance companies just swap out biologics that are working for a patient for a “generic” version. The more you dive into it, the more you’ll realize that a biosimilar IS NOT just a generic form of a biologic. That these new drugs can actually provide a lot of positive benefits, BUT there needs to be regulation around them. This is something that the FDA and our health care system has never dealt with before, since biologics are in a whole drug class of their own.
But first, let’s start at the very beginning… a very good place to start 😉
Are Biosimilars good or are they bad?
So many chronically fabulous patients are on biologics and let’s be honest, biologics can be a scary drug to take! The side effects sometimes can seem like they’re endless. I was talking to a woman who’s 6 year old daughter was diagnosed with JRA at 3 years old. She was first put on Enbrel, but after developing arthritis in her eye, she was switched to Remicade. When she was first put on the biologics the doctor told her mom, it’s not if, it’s when she develops cancer…. What kind of parent or patient wants to hear that?!
Now, that the pharmacy industry is trying to introduce biosimilars in the USA (they’re already available in some other countries) there are concerns whether this will be a good thing or a bad thing.
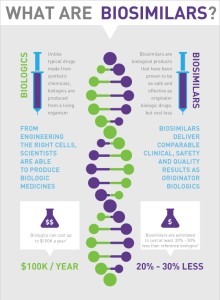
Many, who are pushing for the introduction of these drugs, are basically saying that they’re generic versions of biologics. It would make sense, except…. Biologics aren’t simply a drug. Biologics are extremely complex.
What are biologics?
According to BIO, “A biologic is manufactured in a living system such as a microorganism, or plant or animal cells. Most biologics are very large, complex molecules or mixtures of molecules. Many biologics are produced using recombinant DNA technology.”
The following explanation is from Understanding Biologic Treatments from Healthline.com:
“Most biologics are given by injection. Some are injected under the skin, while others must be injected directly into a vein. One medication, tofacitinib citrate (Xeljanz), is available as a pill taken orally. Biologics provide relief to some patients who do not respond to older drugs. They also have the advantage of fewer side effects. They’re not completely free of side effects, however.
Biologics work by interrupting immune system signals involved in the damage of joint tissue. Many newer drugs target a protein called tumor-necrosis factor (TNF). These drugs are called anti-TNF biologics. Like other DMARDs, biologics affect immune system function. Because of this, they can make a person more susceptible to serious infections. Infections affecting the airways are particularly common. There is also an increased risk of liver damage. The body’s ability to produce new blood cells could also be affected.
Biologics work for more patients because they target specific aspects of the immune system to reduce inflammation in the joints. Any drug that suppresses the immune system carries risks, though. It is important to tell your doctor about any unusual symptoms you experience, such as fever or other symptoms that are not easily explained. For instance, some people may have a dormant infection that can become active after starting biologic therapy. For this reason, it’s important to have a tuberculosis test before taking one of these drugs. People with liver disease may not be eligible to take a biologic drug.”
Want more understanding of the difference between a biologic and a drug? Check out this Everyday Health article.
Bio & Chem Class
In an advocacy meeting I had with a Representative Maher, he made such a great point. Biologics and biosimilars are like comparing chemistry and biology.
In chemistry, 1+1 will always equal 2.
In biology, 1+1 could equal an infinite amount of answers depending on an infinite amount of variables.
Biologics need to be handled a specific way, injected or infused a certain way, shipped in a certain way. One small tweak in the process can ruin a whole batch of the biologic. Biologics are living organisms and are very sensitive.
Right now what the industry is trying to do is to make the biosimilars interchangeable with the biologics.
Just like generic drugs are with brand drugs. There are a few concerns with this approach.
Let’s take this example scenario
The FDA approves Biosimilar A (BSA) as an interchangeable biologic to Biologic A (BA).
1. Since biologics are so complex, there’s no guarantee that BSA will work just as effectively as BA for the patient.
2. There are no delivery standards for these drugs, so sure, maybe the chemical make up of BSA and BA are the same, but if they’re not delivered in the same way they may effect a patient differently. This could be as generic from the way it’s made and delivered to the actual way it’s delivered into the patients body ie. Infusion vs. Self-Injection.
In this example, a patient’s insurance company may decide not to cover the original biologic BA and only cover the biosimilar BSA. Many patients are very afraid of this happening! Now insurance companies do it with generic drugs, so why would these drugs be any different? Some patients spend years and years and years trying to find the right medication to help control their symptoms and with the induction of these new drugs they may be forced to switch to something that, not only isn’t the same drug, but may end up being non-effective!
The point it boils down to is this, biologics and biosimilars are NOT the same and should not be regarded as interchangeable.
Biosimilar Opportunities?
I always looked at biosimilars in a negative light, BUT! There are some really great opportunities on the horizon because of biosimilars as well.
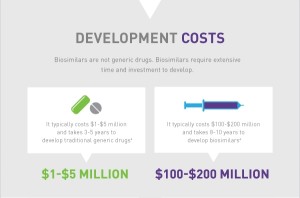
Benefit 1. Right now there is NO competition in the biologic space. That’s why it can easily cost $2,500 per month for a biologic drug. With the induction of biosimilars, there will easily be a 30/40% reduction in the price of these drugs. This WILL create competition in the market and hopefully bring the cost of the biologics down as well.
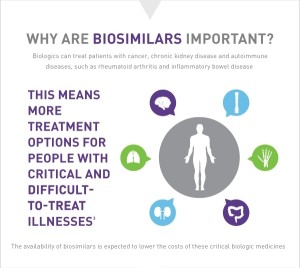
Benefit 2. With the induction of these new drugs, there will now be more treatment options to explore. It’s one thing for the insurance company to mandate you to take one Biosimilar over a biologic, but it’s another to actively choose to try a Biosimilar. Who knows, some of these news drugs may end up being more effective than what we have now. Only time will tell!
Biosimilars can be a great pathway to helping drive down prices in the biologic space and can really offer patients more treatment options. The main issue is that there needs to be legislation surrounding the interchangeability of these compounds and how that will all work. Right now, generics can interchangeably be filled for a brand drug. Because biologics and biosimilars are different than brand vs. generic, there are several states trying to pass and introduce bills to help regulate it.
While in Harrisburg for the Advocacy Day, we talked with our Representatives and Senators to support a bill that would mandate communication between pharmacists and doctors regarding the interchangeability of biosimilars and biologics.
ISSUE: Biosimilar Substitution
In any instance of a Biosimilar product substitution for a biologic medicine, prescriber communication is essential.
The background of the bill:
– Biosimilars are complex genetically engineered products which offer new treatment opportunities for people with forms of inflammatory autoimmune arthritis and other chronic diseases.
– Through special review processes conducted by the FDA some of these Biosimilar products may be deemed to be therapeutically equivalent of interchangeable with an original biologic or reference product.
– In the future, interchangeable biosimilars recognized by the FDA may be substituted for an approved biologic.
– As both biologics and biosimilars are complex treatments requiring careful therapeutic monitoring, pathways for substitution require communication and transparency in all pharmacy transactions.
Our requests were:
– Communication to the patient upon substitution
– Communication to the prescriber within 48 hours of the substitution
– Retention of substitution records for a minimum of 5 years
– Permit a physician to override to substitution where patients are stable on a prescribed biologic
– Biosimilar medications must be approved by the FDA as therapeutic equivalent and interchangeable to original biologic
– Biosimilar medications must have an individualized and unique name noticeably different than the reference biologic.
Everyone we talked to seem to agree that the bill would do a whole heck of a lot of good and there really aren’t any downfalls to the bill. After spending the day talking a lot about biosimilars, I think that their introduction into our health system is going to do a lot of good!
The Arthritis Foundation has a great webinar put on by a doctor on biosimilars. Check it out if you’d like to learn more about it!
The National Psoriasis Foundation also has put out a webinar! Check it out here.
What do you think about biosimilars???
Wishing You A Pain Free Day!